Abstract
Background: Acute myeloid leukemia (AML) is a heterogeneous entity characterized by a myriad of cytogenetic and molecular abnormalities of prognostic relevance. Comprehensive characterization of AML-related genetic abnormalities and associated outcomes has allowed for a genomic classification in AML ( Papaemmanuil et al, NEJM, 2016 ). In this study, we report outcomes of patients with AML (≤ 65 years) enrolled in a clinical trial involving the combination of cladribine, idarubicin, cytarabine (CLIA). We further evaluate survival in frontline treated patients (pts) by specific genomic sub-groups ( Papaemmanuil et al, 2016 ) in this uniformly treated population.
Methods: Induction regimen constituted cladribine 5 mg/m2 IV over 30 minutes on days 1-5, followed by cytarabine (ara-C) 1 g/m2 IV on days 1-5, and idarubicin 10 mg/m2 IV days 1-3. Consolidation consisted of up to 5 more cycles of CLIA. Sorafenib was additionally in FLT3- ITD mutated patients. Pts were enrolled under three cohorts: frontline, secondary AML (s-AML), and relapsed/refractory (R/R). 143 pts have been enrolled, of whom 128 (including 71 out of 73 frontline) had next generation sequencing performed prior to starting treatment. Newly diagnosed and R/R AML pts were classified into specific genomic sub-groups.
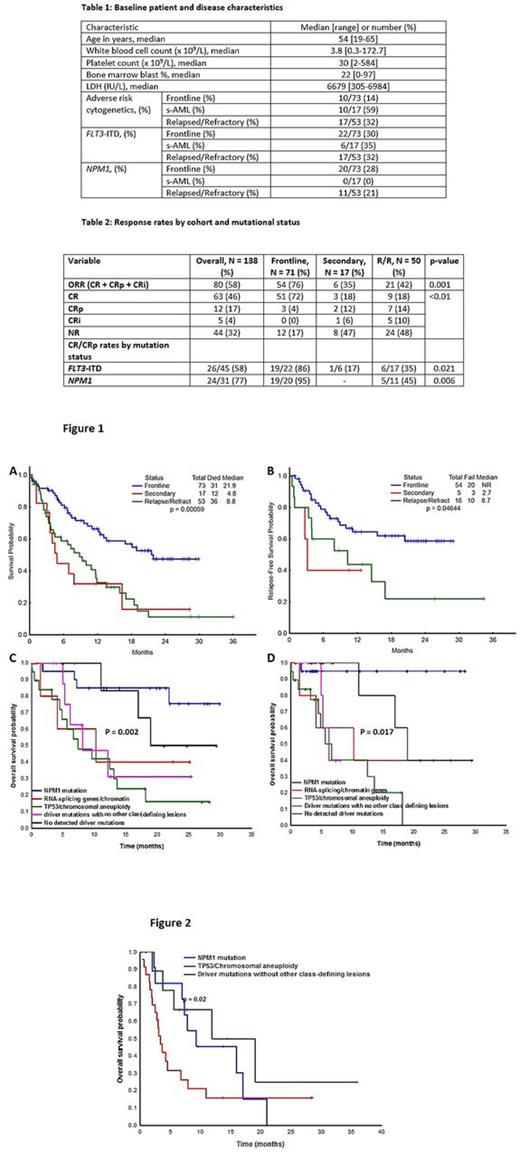
Results: Among the 143 pts enrolled, 73 (51%) pts belonged to frontline, 17 (12%) to s-AML, and 53 (37%) to R/R cohorts, respectively. Baseline pt and disease characteristics are outlined in table 1. None of the patients remain on study; reasons for study cessation included: disease progression / relapse / no response (46%), received SCT (31%), completed (10%), patient choice (2%), death (2%), other (9%). The cumulative 4-week and 8-week mortality rates in the entire cohort were 3% and 9%, respectively. Hundred and thirty eight of 143 (97%) enrolled patients were evaluable for response. Responses by cohort are detailed in table 2. 71 (97%), 17 (100%), and 50 (94%) pts were evaluable for response in frontline, secondary and R/R cohorts; ORRs were 76%, 35%, 42%, respectively (p = 0.001). At a median study follow up of 13.8 (0.3-36) months among survivors, median OS estimates were 21.9, 4.8, and 8.8 months for the frontline, s-AML, and relapsed cohorts, respectively (figure 1A). Median relapse free survival in frontline, secondary, relapsed cohorts were not reached (NR), NR, 5.1 months, respectively (figure 1B). In the R/R cohort, median number of prior therapies received were 2 (1-4). There was an improved OS in the early salvage setting (1st salvage) [vs late salvage (≥2 previous therapies) [10.9 vs. 4.4 months, p = 0.01]. Among the frontline treated patients, the most frequently occurring mutations were FLT3 ITD (33%), NPM1 (28%), RAS (23%), DNMT3A (22%); TP53 mutations were detected in 12%. Segregating patients by Papaemmanuil classification, the most common genomic sub-groups were NPM1 mutation (Group A, n = 20); TP53 /aneuploidy (Group B, n = 19); driver mutations but no detected class-defining lesions (Group C, n = 11); no detected driver mutations (Group D, n = 9); mutated chromatin, RNA-splicing genes (Group E, n = 6), others (n = 6); CR/CRp rates in the groups A, B, C, D, E were 95%, 80%, 50%, 81%, and 77%, respectively (p < 0.01). Subgroup-survival analysis identified Group A and Dto be associated with improved OS when compared to Group B, C, and E (figure 1C). A similar trend was observed even after censoring for transplant (figure 1D). In the R/R setting, Group A (n = 9), C (n = 20), D (n = 11) were most common, with Group C associated with worse outcomes compared to Group A and Group D (Figure 2).
Conclusions: The combination of CLIA constitutes an effective and safe induction regimen in younger AML patients. Outcomes are impressive in the frontline and early salvage setting. Genomic subgrouping identifies high risk AML groups, particularly TP53 /aneuploidy, driver mutations, andchromatin/RNA-splicing genes, in which there exist a critical need for risk-adapted therapeutic approaches.
Kantarjian: Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; ARIAD: Research Funding; Delta-Fly Pharma: Research Funding; Pfizer: Research Funding; Novartis: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Daver: Pfizer Inc.: Consultancy, Research Funding; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Otsuka America Pharmaceutical, Inc.: Consultancy; Karyopharm: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Incyte Corporation: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Jazz: Consultancy; Bristol-Myers Squibb Company: Consultancy, Research Funding; Kiromic: Research Funding; Immunogen: Research Funding. Pemmaraju: Novartis: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; LFB: Consultancy, Honoraria, Research Funding; Stemline: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria. DiNardo: AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding. Verstovsek: Seattle Genetics: Research Funding; Galena BioPharma: Research Funding; Bristol Myers Squibb: Research Funding; Astrazeneca: Research Funding; Celgene: Research Funding; Lilly Oncology: Research Funding; Blueprint Medicines Corp: Research Funding; Bristol Myers Squibb: Research Funding; NS Pharma: Research Funding; Astrazeneca: Research Funding; Galena BioPharma: Research Funding; Blueprint Medicines Corp: Research Funding; CTI BioPharma Corp: Research Funding; Promedior: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Roche: Research Funding; Incyte: Research Funding; Roche: Research Funding; Pfizer: Research Funding; CTI BioPharma Corp: Research Funding; Gilead: Research Funding; Promedior: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Genentech: Research Funding; Pfizer: Research Funding; Celgene: Research Funding; Lilly Oncology: Research Funding. Jain: Genentech: Research Funding; Incyte: Research Funding; BMS: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cortes: Pfizer: Consultancy, Research Funding; Sun Pharma: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Teva: Research Funding; ImmunoGen: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal